
Home » Products » Analytical Solutions » Automated Reactors & IN SITU » Mettler Toledo Reaction Calorimeters
Reaction Calorimeters
For safety studies
Mettler Toledo Reaction Calorimeters
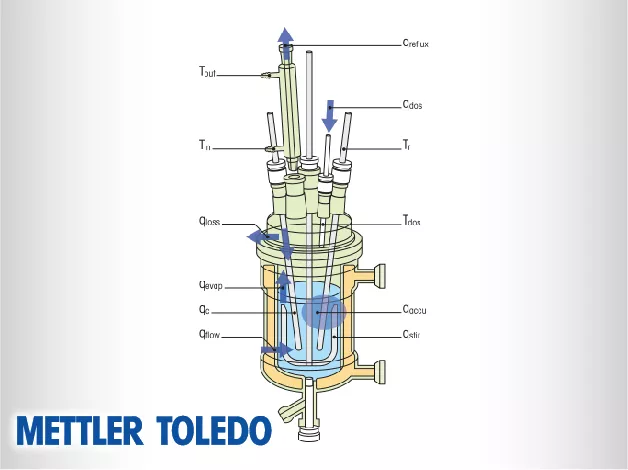
Mettler Toledo reaction calorimeters, led by the industry-standard RC1mx and HFCal systems, are high-precision technical instruments designed to measure the heat released or absorbed during a chemical reaction. These systems function as automated lab reactors that provide essential thermochemical data, such as heat flow, enthalpy, and heat capacity, under process-like conditions.
By accurately quantifying the energy changes within a vessel, these calorimeters enable chemical engineers and safety experts to determine the potential for thermal runaway and design robust cooling systems. This technical foundation is critical for ensuring that chemical processes are both efficient and inherently safe when scaled from the laboratory to industrial production.
The RC1mx as the Global Standard for Reaction Calorimetry
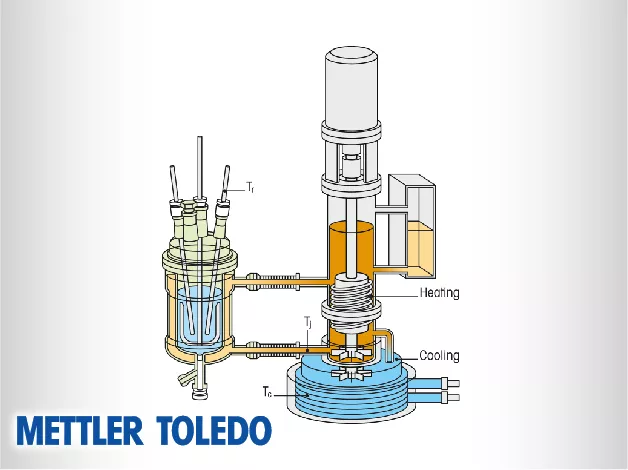
The RC1mx is technically recognized as the gold standard for reaction calorimetry in the chemical and pharmaceutical industries. It utilizes a highly sophisticated thermostat system that can precisely control temperatures while simultaneously measuring the heat flow generated by the chemistry inside the vessel. This system is engineered to simulate industrial reactor conditions, allowing researchers to gather thermodynamic data that is directly applicable to large-scale manufacturing. The RC1mx provides a robust platform for determining the thermal risks associated with chemical processes, ensuring that scale-up is managed with data-driven safety margins.
Principles of Heat Flow Calorimetry (HFCal)
Technically, Heat Flow Calorimetry (HFCal) measures the heat of a reaction by monitoring the temperature difference between the reaction mass and the jacket of the vessel. The HFCal technology integrated into systems like EasyMax and OptiMax allows for the real-time calculation of the heat transfer coefficient ($U$) and the total heat flow ($q$). This enables scientists to determine the reaction enthalpy and the power of the reaction throughout its entire duration. By quantifying how much energy is released and at what rate, HFCal provides the necessary parameters to design safe and efficient industrial cooling strategies.
Determining Enthalpy and Heat Capacity
A critical technical function of reaction calorimeters is the determination of specific thermochemical properties such as enthalpy ($\Delta H$) and heat capacity ($C_p$). During a calibrated experiment, the system measures the total energy released during a chemical transformation. This data is essential for calculating the "Cooling Capacity" required in a production-scale reactor. By understanding the heat capacity of the mixture, engineers can predict how the temperature will change in response to energy inputs or losses, which is vital for maintaining control over highly exothermic or sensitive chemical pathways.
Safety Assessment and Adiabatic Temperature Rise
One of the most important technical applications of reaction calorimetry is the assessment of process safety through the calculation of the Adiabatic Temperature Rise ($\Delta T_{ad}$). By measuring the heat of reaction and the heat capacity, the calorimeter allows scientists to predict the maximum temperature the reaction could reach if the cooling system were to fail. This data is used to determine the "Time to Maximum Rate" (TMR) under adiabatic conditions, which is a key parameter for emergency relief system design and for establishing safe operating envelopes in chemical plants.
Scale-up Optimization and Pilot Plant Simulation
Reaction calorimeters are technically designed to bridge the gap between laboratory discovery and industrial production. They allow for the simulation of large-scale reactor dynamics, including mixing efficiency, mass transfer, and dosing-controlled heat release. By using a calorimeter to optimize these parameters, chemical engineers can identify potential bottlenecks or safety hazards before they occur in a pilot plant. This predictive capability reduces the risk of batch failures and ensures that the transition to large-scale manufacturing is both economically viable and technically sound.
Integration with iC Software for Automated Data Analysis
The RC1mx and HFCal systems are supported by the iC Software suite, which provides advanced tools for automated data processing and thermochemical calculations. Technically, the software converts raw sensor data into meaningful heat flow curves and energy balances. It can automatically calculate the baseline of the reaction and integrate the area under the curve to find the total heat of reaction. This digital integration simplifies the complex math involved in calorimetry, allowing researchers to quickly visualize thermal trends and generate comprehensive safety reports for regulatory compliance.
Real-Time Monitoring and Process Analytical Technology (PAT)
Reaction calorimeters function as a core component of Process Analytical Technology (PAT). They can be integrated with in situ probes such as ReactIR (FTIR) or ReactRaman to correlate thermochemical data with molecular changes. This technical synergy allows scientists to see not only how much heat is being released but also which specific chemical step is responsible for the energy change. Understanding the relationship between chemical conversion and heat release is essential for developing robust processes that are resistant to variations in raw materials or environmental conditions.
Versatility for Diverse Chemical Environments
Mettler Toledo calorimeters are engineered for technical versatility, with a wide range of vessel types and materials, including glass, stainless steel, and Hastelloy. These vessels can operate under vacuum or high-pressure conditions and across a broad temperature range. This flexibility allows for the study of diverse chemistries, from low-temperature organometallic syntheses to high-pressure hydrogenations. The robust design ensures that the sensors and heating/cooling systems maintain their calibration and precision even in aggressive chemical environments or during prolonged multi-day experiments.
Read more about Mettler Toledo Reaction Calorimeters







.webp)

.webp)