Mobile Scalable Bio-Decontamination System
For Cleanrooms and GMP Facilities
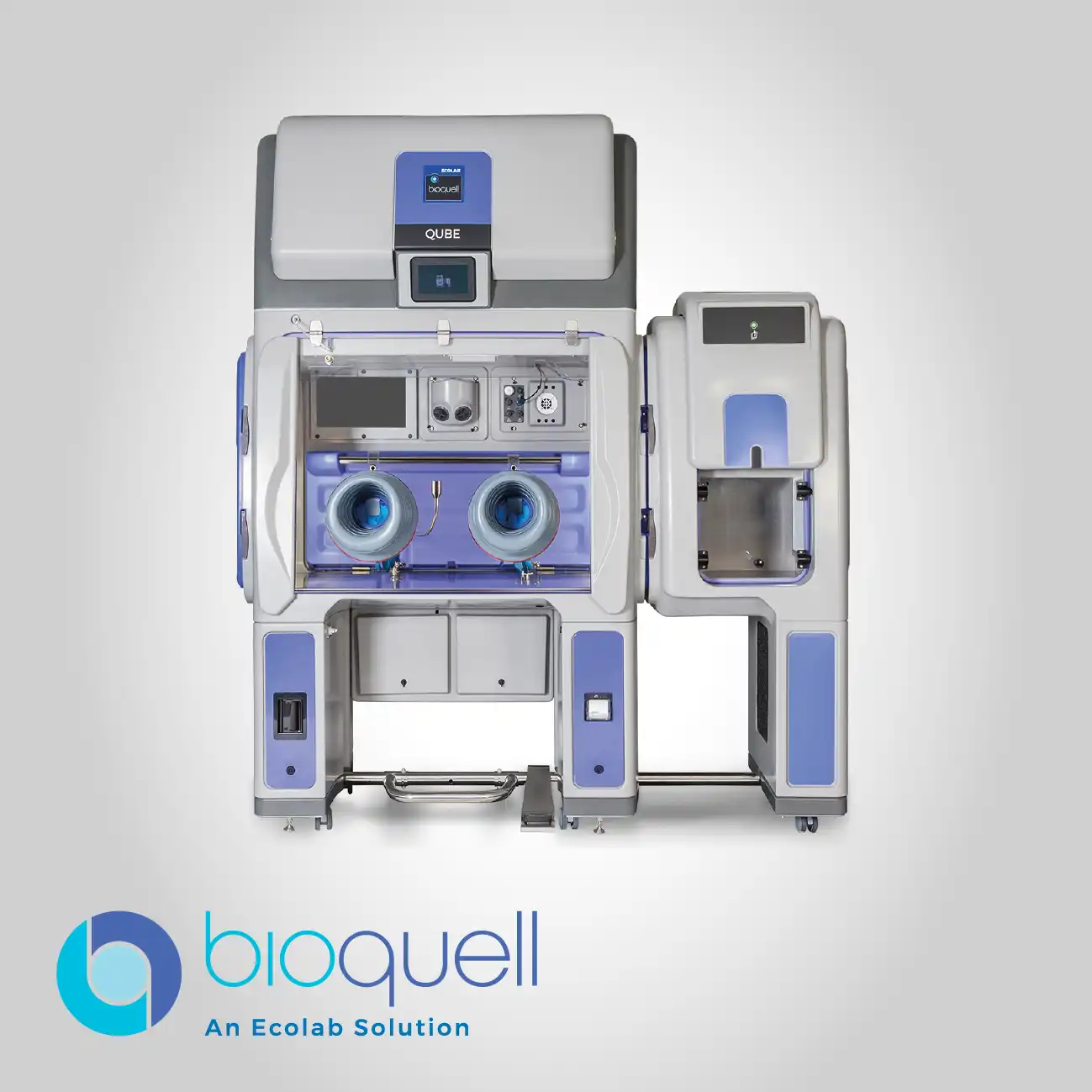
Bioquell ProteQ
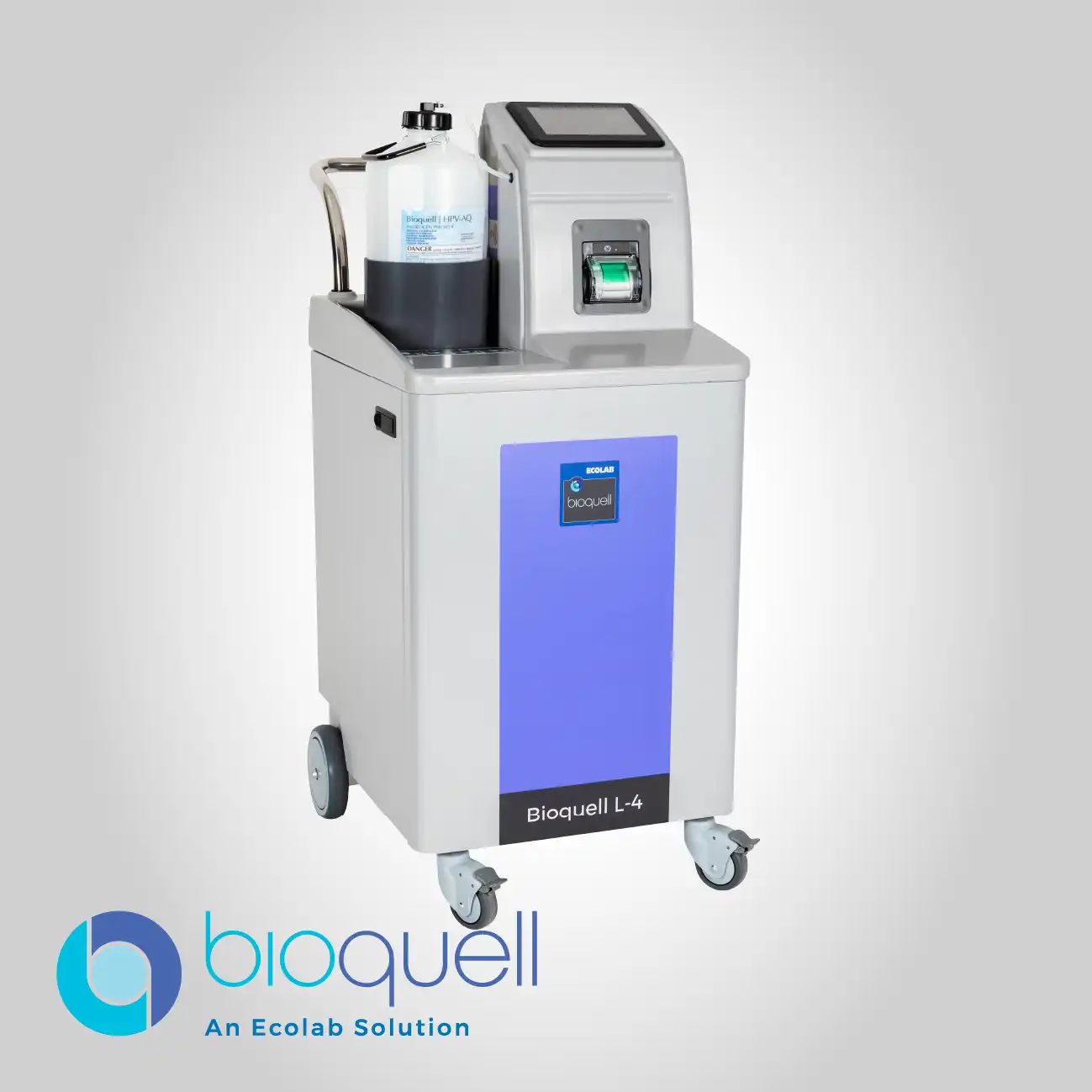
The core function of this unit is to deliver a potent 6-log sporicidal kill on all exposed surfaces using Hydrogen Peroxide Vapor technology. This high level of efficacy is critical for maintaining regulatory standards in high-risk zones, providing a robust solution against a broad spectrum of microbial contaminants and ensuring facility readiness after maintenance or production campaigns.
Designed with user-friendliness in mind, the system's simple setup protocol is facilitated by its all-in-one housing, which eliminates the need for separate external components. Its mobility allows it to be easily transported and deployed across different operational areas, enhancing facility-wide utilization and maximizing asset efficiency within complex laboratory and production infrastructure.
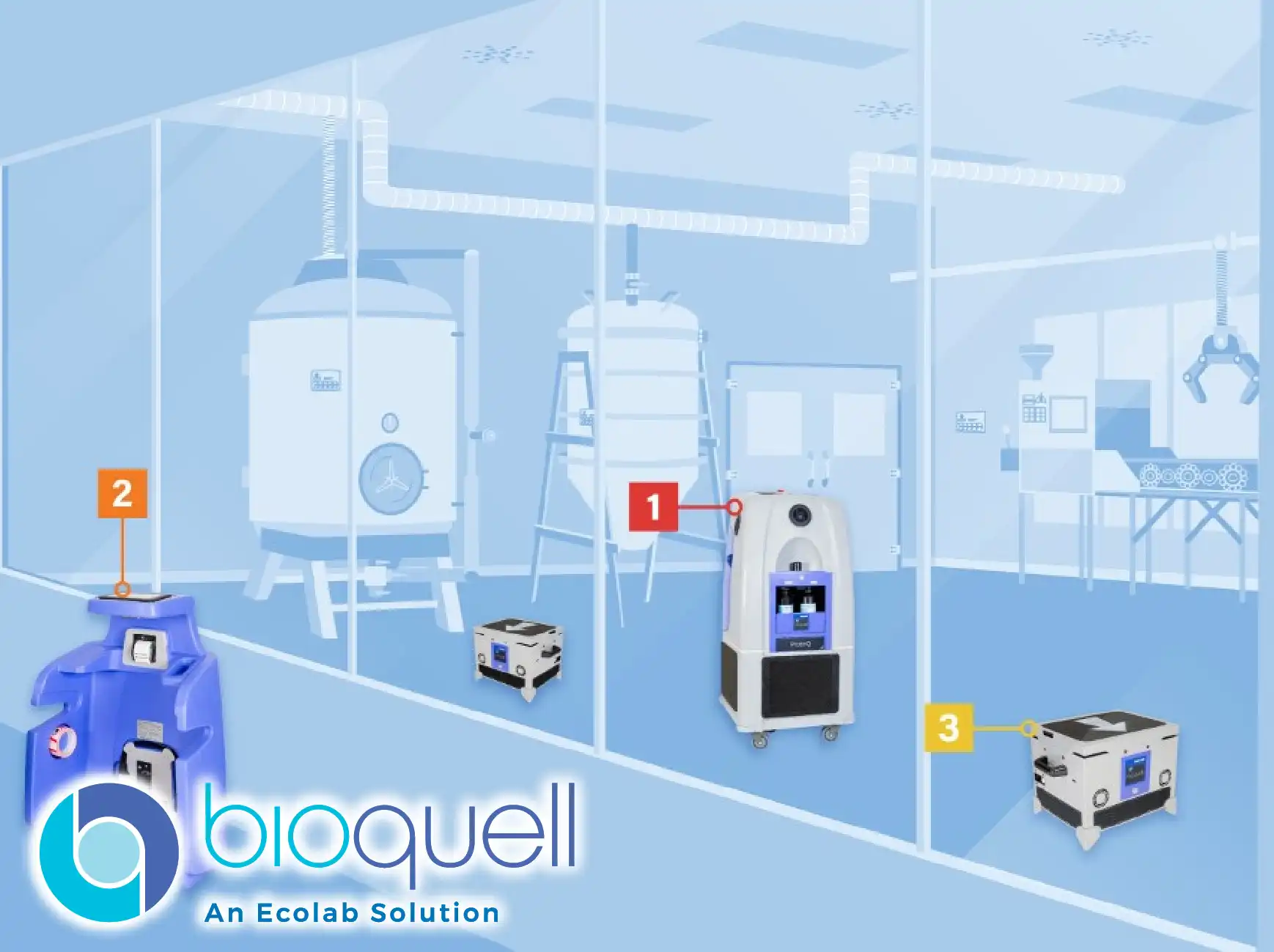
The challenge of extended facility Downtime due to traditional cleaning methods is addressed by this system's swift decontamination cycles and streamlined setup process. Its mobile design allows for targeted treatment of specific zones, minimizing the impact on adjacent, ongoing operations and dramatically improving overall facility throughput.
Achieving consistent regulatory Compliance across varied room geometries often proves difficult; however, the scalable nature of this system ensures effective vapor distribution regardless of room volume. This consistent performance provides auditable validation data, mitigating the risk of failed regulatory inspections and batch loss.
The integration of Wireless communication technology resolves the logistical complexities associated with extensive cable management and remote monitoring, particularly in segregated or high-containment areas. This feature enables safe, remote operation and real-time cycle data tracking, increasing operator safety and procedural control.
Traditional systems often require significant manual intervention for setup and teardown, introducing the risk of Human Error; the all-in-one housing and built-in aeration components simplify the entire process. This mechanical integration reduces dependency on complex manual configuration, ensuring greater repeatability and consistency in every cycle.
The modular design, which accommodates built-in and optional Aeration capabilities, tackles the common issue of lengthy post-decontamination return-to-use times. By accelerating the removal of the sterilant from the environment, the system drastically compensates for the facility's idle period, boosting production capacity.
Managing contamination risks in diverse environments, from large manufacturing suites to smaller Bio-Safety labs, requires a versatile solution. This unit's adaptability allows a single platform to be utilized across multiple operational types, consolidating training and maintenance procedures.
For facilities dealing with frequent changeovers, the system offers a high degree of Repeatability in its decontamination parameters. This is crucial for validation masters, as identical cycle performance is guaranteed, simplifying documentation and reducing the validation burden associated with routine use.
The self-contained nature of the system's Integration ensures that all necessary processing and control elements are housed within one frame. This feature eliminates compatibility issues between separate components and ensures system function remains reliable, lowering the total cost of ownership through simplified maintenance.
Click here to know more about Bioquell products and solutions