Integrated H₂O₂ Vapor System
For Total Protocol Automation
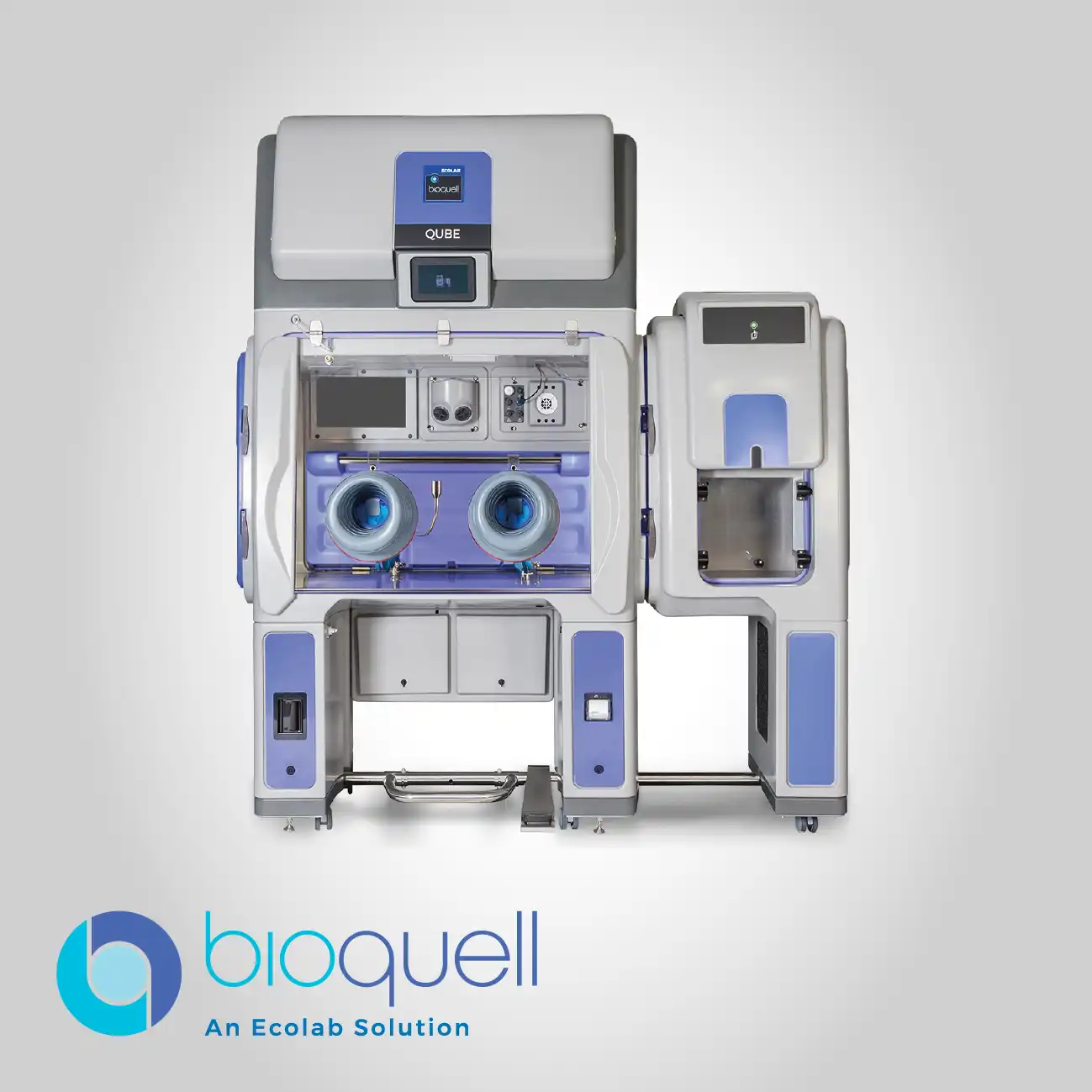
Bioquell IG-2
The IG-2 system is purpose-built to facilitate the total, consistent integration of Hydrogen Peroxide Vapor technology directly into existing facility protocols and critical processing equipment. Its design ensures that the vapor bio-decontamination process becomes a seamless and reliable part of the workflow, offering expert support to guarantee the right system setup and consistent performance. This approach transforms decontamination from a separate, often manual, task into an automated, inherent feature of the operational environment, especially for high-throughput and small-volume spaces.
This system is specifically optimized for integration into highly automated or restricted environments, including Restricted-Access Barrier Systems (RABS), filling lines, and complex robotic enclosures. By enabling complete system integration, it removes the need for cumbersome external equipment setups, significantly enhancing workflow continuity and reducing the potential for contamination during transfers.
The consistent application of H₂O₂ Vapor is key to its functionality, providing a 6-log sporicidal kill necessary for meeting stringent regulatory standards in pharmaceutical and biomedical facilities. This integration-focused model provides a pathway for automating compliance in critical areas like material airlocks and small volume rooms, where manual processes are prone to variability.
Achieving consistent decontamination in high-speed, automated Filling Lines is a major operational constraint; the system allows the H₂O₂ Vapor process to be fully integrated into the line's sequence. This capability ensures that the surrounding environment and internal surfaces are sterilized automatically without stopping the overall production flow.
The design focuses on Total System integration, directly solving the problem of relying on external, temporary decontamination systems that can introduce logistical hurdles and connection failures. By embedding the technology, the entire process becomes more reliable and ready-to-use.
For facilities using Robotic Enclosures, which are often inaccessible and complex, surface sterilization is difficult. The system enables the introduction of vapor into these enclosures as a standard, repeatable protocol, ensuring that the automated equipment itself does not become a source of contamination.
In RABS and Isolator setups, the critical pain point is maintaining the barrier while performing high-level sanitization; this integrated system allows for remote activation and control. This preserves the restricted-access integrity and improves operator safety by minimizing interaction with the sterilant.
The system's application in Small-Volume Rooms addresses the issue of over-gassing and lengthy aeration times associated with non-optimized generators. Its integrated control ensures precise delivery and removal of the vapor tailored to the specific volume, speeding up the overall cycle time.
The provision of expert Support for system setup is critical, as incorrect installation can lead to validation failure. This dedicated service ensures that the integration is executed correctly from the start, mitigating the financial risk of non-compliant deployment.
The system facilitates the continuous and Consistent application of the vapor process, which is essential for maintaining a validated state. This automation reduces the dependence on manual operator variability, ensuring the same successful sporicidal kill in every cycle.
Integrating H₂O₂ Vapor into Material Airlocks as part of a fixed protocol solves the problem of unreliable transfer sanitation. By making the vapor cycle inherent to the airlock function, the system enforces a strict biosecurity checkpoint for all incoming and outgoing materials.
Click here to know more about Bioquell products and solutions